Aspartic Acid at Ph 3
Amino acid dating is a dating technique used to estimate the age of a specimen in paleobiology molecular paleontology archaeology forensic science taphonomy sedimentary geology and other fields. Errors in amino acid placement do occur and can lead to cell death in some instances.
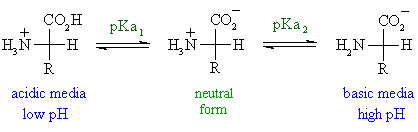
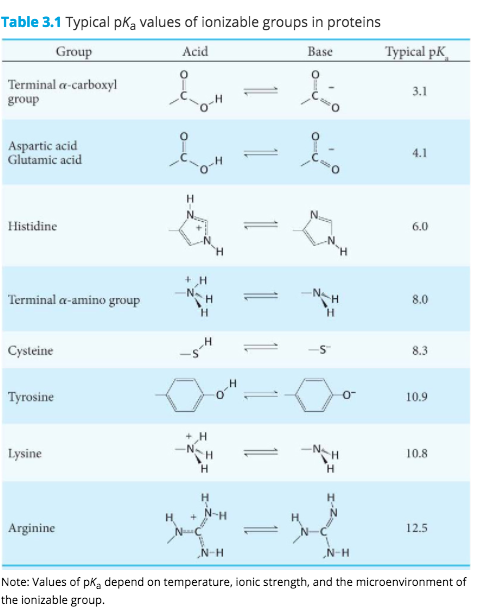
3 pKx is the negative of the logarithm of the dissociation constant for any other group in the molecule.
. Subtracting an the weight of a mole of water 18gmol yields the molecular weight of the. Zusätzlich ist jedoch die zweite Carboxygruppe deprotoniert darum wird in der Biochemie statt L-Asparaginsäure oft die Bezeichnung L-Aspartat verwendet. Lacido aspartico come la maggior parte degli amminoacidi è soggetto a stereoisomeria.
Other mechanisms include minimization of solvent accessibility of acidic residues or binding of metal cofactors. Glutamine or Glutamic acid. Prin prefixele di tri etc se arată numărul de grupe amino și carboxil iar poziția relativă a două grupe funcționale se precizează cu literele grecești α β γ δ ε în care acidul este α dacă grupările.
Including but not limited to citric acid glycolic acid lactic acid malic acid mandelic acid. In biomedical sciences and is a science writer educator and consultant. Always keep in mind structure gives function.
To use our peptide calculator mass properties enter the sequence or the amino acid using 1-letter or 3-letter amino acid codes and our calculator will provide the following physico-chemical properties of the sequence. Learn about our Editorial Process. 2 pKb is the negative of the logarithm of the dissociation constant for the -NH3 group.
The amino acid name the structure the pKa of ionizable hydrogens and both the 3-letter and 1-letter shorthand. Helmenstine holds a PhD. Bulk Drug Substances Nom inated for U se in Com pounding Under Section 503B.
She has taught science courses at the high school college and graduate levels. Alpha1- proteinase inhibitor human I. In most acid stable proteins such as pepsin and the soxF protein from Sulfolobus acidocaldarius there is an overabundance of acidic residues which minimizes low pH destabilization induced by a buildup of positive charge.
Formulations containing alpha hydroxy acids alone or in combination at concentrations of greater than 30 andor with a pH lower than 30 except when sold to be applied to warts corns or calluses. Asaragine or Aspartic acid. The alkoxide will then be attacked by the electrophilic Au-OH ads to generate OHC-Ph-COO and H 2 O step 4 in Fig.
For 4-HMBA the proton of the hydroxyl group can be removed at a moderately positive potential in the alkaline electrolyte and form the alkoxide OCH 2-Ph-COO step 3 in Fig. Updated on March 06 2017. Updated January 27 2022.
Wie die anderen Aminosäuren liegt Asparaginsäure im Körper normalerweise zwitterionisch vor. Each molecule contains a central carbon C atom called the α-carbon to which both an amino and. Aminoacizii se denumesc folosind cuvântul acid urmat de amino și numele acidului corespunzător.
All biological tissues contain amino acidsAll amino acids except glycine. Additionally the tool includes a hydrophobicity calculator a net charge calculator at different pH isoelectric point calculator and the hydrophilicity ratio. Lide Handbook of Chemistry and Physics 72nd.
The molecular weights above are those of the free acid and not the residue which is used in the claculations performed by the Peptide Properties Calculator. That is a daunting. Siccome i due gruppi carbossilici hanno costanti di dissociazione acida differenti è possibile identificare il punto isoelettrico a pH pari a 285.
Amino acid any of a group of organic molecules that consist of a basic amino group NH2 an acidic carboxyl group COOH and an organic R group or side chain that is unique to each amino acid. Of the Federal Food Drug and Co smetic Act. Asparaginsäure abgekürzt Asp oder D ist in ihrer natürlichen L-Form eine der proteinogenen α-Aminosäuren.
This technique relates changes in amino acid molecules to the time elapsed since they were formed. Amino acids are the building blocks of. 1 pKa is the negative of the logarithm of the dissociation constant for the -COOH group.
In a specialized case. 4 pl is the pH at the isoelectric point. The term amino acid is short for α-amino alpha-amino carboxylic acid.
Most biochemistry courses will require you to know the following. La forma zwitterionica viene invece raggiunta a pH acidi quando il gruppo amminico è protonato e solo uno dei due gruppi carbossilici è deprotonato. Alphadolone or its salts.
Lipids and fats proteins and polypeptides carbohydrates nucleic.
Solved 3 For Aspartic Acid At Ph 7 What Is The Ratio Of Chegg Com
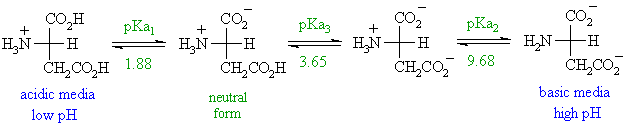
No comments for "Aspartic Acid at Ph 3"
Post a Comment